What Is That Conical Thing in a Chemistry Lab? A Guide to the Erlenmeyer Flask
Almost every chemistry lab contains a conical glass flask that students point at and ask about. That “something conical in a chemistry lab” is usually the Erlenmeyer flask, also called a conical flask or titration flask.
People often first mefeels et it in a school lab while measuring acids and bases or swirling colorful solutions. At first, it like just another piece of laboratory glassware. Once you understand why its body is conical and its neck so narrow, it starts to make a lot more sense.
Erlenmeyer Flask: The Conical Workhorse of the Lab
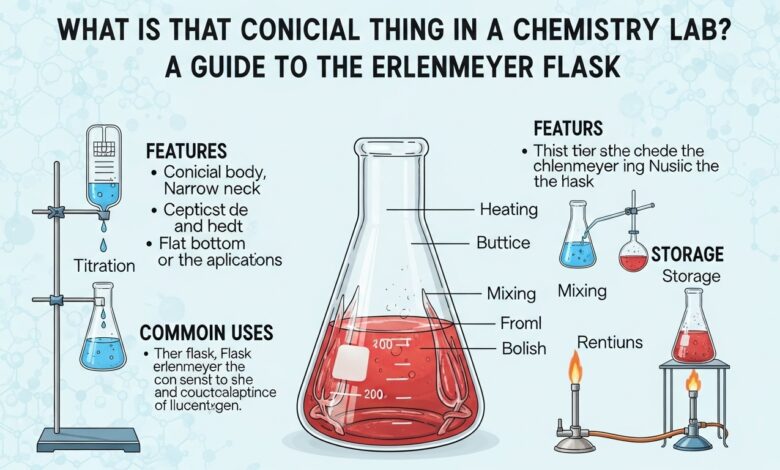
An Erlenmeyer flask is a piece of laboratory glassware with a flat bottom, a conical body, and a short cylindrical neck. The wide base lets the flask sit firmly on a bench or hot plate, while the narrowing upper section collects the liquid in the middle when you swirl it. The neck is narrow enough to accept a stopper, cotton plug, or parafilm so that you can limit evaporation and stray dust.
Many teachers and lab manuals also call it a conical flask or a titration flask. This design was introduced in the nineteenth century and is named after the German chemist, Emil Erlenmeyer, who named the design after his name. This flask is found today in schools and industrial laboratories, and microbiology labs throughout the United States.
Erlenmeyer flasks are commonly manufactured out of borosilicate glass able to withstand heating/cooling conditions compared to normal glass. Certain ones are made of plastic, particularly for sterile microbiology work.
The side has volume markings that are only approximations of the amount of liquid in it. A volumetric flask, burette, or pipette should be used in case you require an accurate amount of the volume.
Why Chemists Trust the Conical Flask Design
The design of the Erlenmeyer flask solves several practical problems that come up in basic lab work.
Stable base for heating and cooling
The wide base keeps the flask steady on a hot plate or wire gauze over a burner. A tall cylinder tips easily. A conical flask with a broad bottom resists accidents when you swirl or move the vessel and spreads heat over the liquid.
Sloped sides for swirling without spills
The gradual taper of the walls lets you swirl liquids vigorously without splashing over the side. When you hold the neck and move your wrist in a circular motion, the liquid climbs the walls and falls back toward the center. That motion mixes reagents and keeps them in the flask.
Narrow neck for better control
There are a number of advantages to the narrow neck. It may be capped with a stopper to minimize evaporation, tubing or thermometer may be attached, or a filter funnel may be securely held in it.
Since the neck is narrow, it causes less exposed area to the air. This is necessary in case of dealing with volatile solvents or solutions that must not take up the moisture or CO2 of the air.
Put differently, this flask is not only useful on the bench but also in books, due to the wide bottom, conical sides, and thin sides on the neck.
Common Uses of the Erlenmeyer Flask
Chemists and students reach for the Erlenmeyer flask in many situations. These are the uses you see most often.
Solution mixing and dissolving
The conical flask has a reputation as the mixer of the lab. You can add a solid and a solvent, grip the neck, and swirl to dissolve crystals or powder. The design holds the liquid inside while the swirling motion speeds up mixing and saves time at the bench.
Titration work under a burette
When you imagine the titration apparatus in any general chemistry lab, you probably imagine a burette gradually dripping solution into a conical flask on a white tile. The shape of conical shape enables you to swirl the flask during the titration round without splashing, so that every drop of the burette is mixed with the solution.
When using an indicator, only one drop can be used in an acid-base titration to show the endpoint. The solution is concentrated in the center due to the conical form, which makes this change in color easier.
That is why numerous voice-search questions are formulated as, Why is a conical flask used in titration, rather than a beaker? Why is a flask used under the burette?
Safe heating and boiling in a conical flask
Chemists do not always prefer a beaker over an Erlenmeyer flask in cases of gentle heating of solutions. It has a small neck that reduces the speed of evaporation, a stable bottom that does not go off the hot plate, and splashes are small in a conical shape. This flask can be used to boil some water or to warm a reaction mixture, or even to carry out a simple recrystallization.
Nevertheless, hot glassware should be handled. Wear tongs or heat-proof gloves, and do not do it too hot or too cold. Brosilicate glass stands heat better than regular glass, but any glass can break when it gets unequally hot or gets immersed in cold water when hot.
Filtration and recrystallization
In gravity filtration, a funnel is placed in the neck of an Erlenmeyer flask. Pour the suspension into the filter paper, but the neck of the flask holds up the neck of the funnel, and the filtrate is directed to the bottom. In the case of vacuum filtration, a heavy-walled filter flask with a side arm attached to the vacuum line is designed. The funnel is stacked at the top, and the seal of the system is covered with a filter paper.
In recrystallization, chemists place an impure solid in a solvent in an Erlenmeyer flask. The resulting crystals are filtered after the mixture is heated and then left to cool down. The conical shape of the flask maintains the movement of the solution during cooling and draws the crystals towards the centre.
Short-term storage and transport
The Erlenmeyer flask also serves as a temporary container for solutions between steps. You might store a prepared buffer, a reaction mixture that still needs workup, or a sample that will move from one instrument to another. A stopper or piece of parafilm over the neck protects the contents from dust and slows evaporation during that short period.
Role in Biology and Microbiology
A conical flask can find application in other fields besides chemistry. It plays an important role in microbiology and cell culture labs in the United States.
Microbiologists are used to cultivating bacteria, yeast, and others in Erlenmeyer flasks, which are supplied with either cotton plugs or vented lids. These flasks are conical and have a flat bottom, thus making them suitable for shaking incubators. The platform rotates and pushes liquid and cells around the walls, and fresh medium flows across the surface and supplies the culture with oxygen.
Enzymes, water quality, and small-scale fermentation studies are frequently done in conical flasks in biochemistry and environmental laboratories. The design features that help in mixing and titration in chemistry are the same elements that are useful in monitoring pH, dissolved oxygen, and other biological parameters over time.
Erlenmeyer Flask vs Beaker and Volumetric Flask
The most commonly asked question by students is why there are so many flasks and beakers on a lab shelf. The decision is important in terms of safety or accuracy, or both.
The beaker is straight-sided and has a large and open mouth with a pouring lip. It is best stirred with a rod, to heat large bodies of water or to take approximate measurements when it is not necessary to be very accurate. It is spilled and evaporated more easily by its open top, though. Using a beaker that has been swirled frequently leaves droplets on the counter.
A volumetric flask is long-necked and bears one calibration mark on the bottom, which is round. The filling to such a mark gives a very accurate volume. Volumetric flasks are used by chemists in preparing standard solutions used in quantitative work. They are not made to boil, mix heavily, or filter.
It is between the Erlenmeyer flask. Its graduations are not fine, and therefore it is not employed in the measurements of volumes. Rather, apply it in cases where you have to mix in a swirling manner, heat, title, or avoid splashing solutions. When vigorous movement or reaction and possibly fizz or bump are involved in the work, the conical flask is the preferred choice.
How to Choose and Use a Conical Flask Safely
Good results in the lab depend on matching the glassware to the job and handling it with care.
The right material for your flask
Borosilicate glass is the standard for general chemistry work. It resists most common acids and bases and handles heating cycles in a routine teaching lab or research lab. Plastic Erlenmeyer flasks make sense when you need sterile, disposable vessels, for example, in microbiology or when glass breakage would be too risky.
When you select plastic, you still check how that material behaves with your solvent, your disinfectant, and your autoclave or sterilization method. Some plastics soften at high temperatures or absorb certain organic solvents.
Best volume for the job
Lab suppliers sell Erlenmeyer flasks in many sizes, from tiny 25 or 50-milliliter flasks up to several liters. In practice, most teaching labs rely on 125, 250, and 500 milliliter sizes. As a rule of thumb, filling a flask up to about half or two-thirds of its capacity gives enough space for swirling and boiling without overflow.
Safe handling, heating, and cleaning
Safe handling starts with inspection. Before you pour chemicals, you look for cracks, chips at the rim, or cloudy patches that hint at stress. Damaged glassware belongs in the discard bin, not on the bench.
During heating, you place the flask on a suitable surface such as a hot plate or wire gauze, rather than directly in a strong flame. You swirl gently as the liquid warms and keep your face and hands out of the vapors. When you finish heating, you let the flask cool gradually in air or on a heat-resistant pad.
The next step is cleaning. Rinsing with water, adding a lab detergent, and using a bottle brush help remove residues from the walls and neck. Microbiology labs often finish with an autoclave cycle to sterilize the flasks before reuse.
FAQs
What is the conical glass thing in a chemistry lab called?
Most of the time, that vessel is an Erlenmeyer flask, also called in many classrooms a conical flask or titration flask.
Why do chemists use a conical flask in titration instead of a beaker?
The conical body lets you swirl without spilling, and the narrow neck keeps drops from the burette inside the vessel.
Can you heat liquids in an Erlenmeyer flask?
Yes, you can heat liquids in a borosilicate Erlenmeyer flask on a hot plate or over a suitable burner. You avoid sudden temperature changes and do not let the flask run dry.
Is an Erlenmeyer flask good for storing chemicals?
It works for short-term storage during an experiment, especially with a stopper or parafilm on the neck. For long-term storage of reagents, chemical suppliers and lab safety courses recommend bottles designed specifically for storage.
What size Erlenmeyer flask should I buy for a small lab or home setup?
For general use, many people choose 250 and 500 milliliter flasks first, then add smaller or larger sizes as they gain experience.